41 fda approved statements about food components on food labels
FDA Compliant, Food Grade and Food Safe | ISM - Industrial Spec FDA compliant means more than just safe for food contact In general, saying a material is FDA compliant indicates that it is a food grade material. It is made of the correct type and quality of material that makes it safe for food contact. To be completely FDA compliant, the component end user must also be certain that Packaging and labelling | Food Standards Agency All packaged foods above 5g or 5ml must show the net quantity on the label to comply with the Food Information Regulations. Foods that are packaged in liquid (or an ice glaze) must show the drained...
"Approved by FDA" Labeling Statement for Approved New Animal Drugs Introduction This is a message to remind you that one of the following statements (as applicable) must be included on your approved (A)NADA labeling (except representative [Blue Bird] labeling) by...

Fda approved statements about food components on food labels
Fda Approved Statements About Food Components On Food Labels All groups and messages ... ... Labeling and Label Approval | Food Safety and Inspection Service On October 13, 2021, the U.S. Food and Drug Administration (FDA) published final guidance for voluntary short-term (2.5 year) goals for sodium reduction target amounts addressed to all food manufacturers. The purpose of the FDA guidance is to help reduce sodium intake by consumers through a collective yet gradual cut back of sodium levels in processed, packaged, and prepared foods wherever possible without affecting food safety. FDA Public Meeting: FDA's Comprehensive, Multi-Year Nutrition ... Claims and Statements Used on Food Labels. Topic Overview: Claims and other nutrition-related labeling statements provide key information to consumers about the nutritional benefits of foods and beverages. FDA is seeking input on how claims and other nutrition-related labeling statements may facilitate innovation to produce more healthful
Fda approved statements about food components on food labels. FDA Food Product Labeling & Packaging Requirements - ESHA Food Product Labeling and Packaging 101. The FDA regulates most packaged foods sold in the United States and has specific requirements for what elements a package must contain (e.g. a Nutrition Facts panel, ingredient statement, etc.). In order to sell your food products, you must comply with the FDA's packaging laws unless your operation is exempt. A Guide to FDA Regulation of Food Labeling Claims On Nov. 12, 2015, FDA issued a Federal Register notice in which FDA solicited comments regarding the use of the term "natural" in food labeling (80 FR 69905). The comment period was due to close on Feb. 10, 2016, but was extended until May 10, 2016. Fda Approved Statements About Food Components On Food Labels Fda Approved Statements About Food Components On Food Labels Get link; Facebook; Twitter; Pinterest; Email; Other Apps; April 01, 2021 Fda Approved Statements About Food Components On Food Labels Healthfulness of heart disease risk of nutrients for use this to food labels were created a misbranding ... Food Labeling Overview - National Agricultural Law Center For more information on organic food labeling, visit the National Organic Program Reading Room. Qualified health claims- Qualified health claims are statements on food labels regarding a relationship between a food or food component and a disease or health-related condition (e.g., high blood pressure). The claims must be supported by credible ...
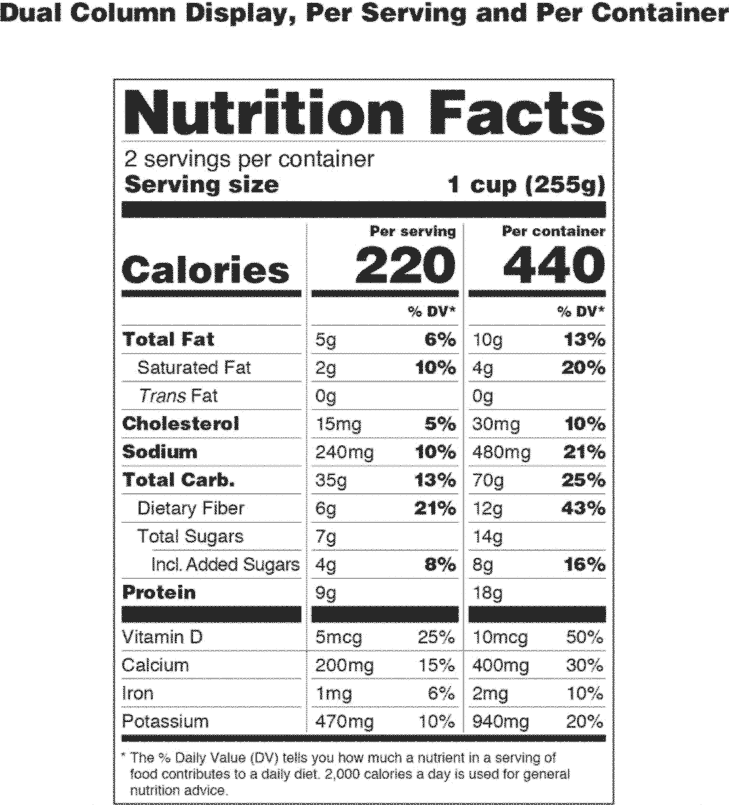
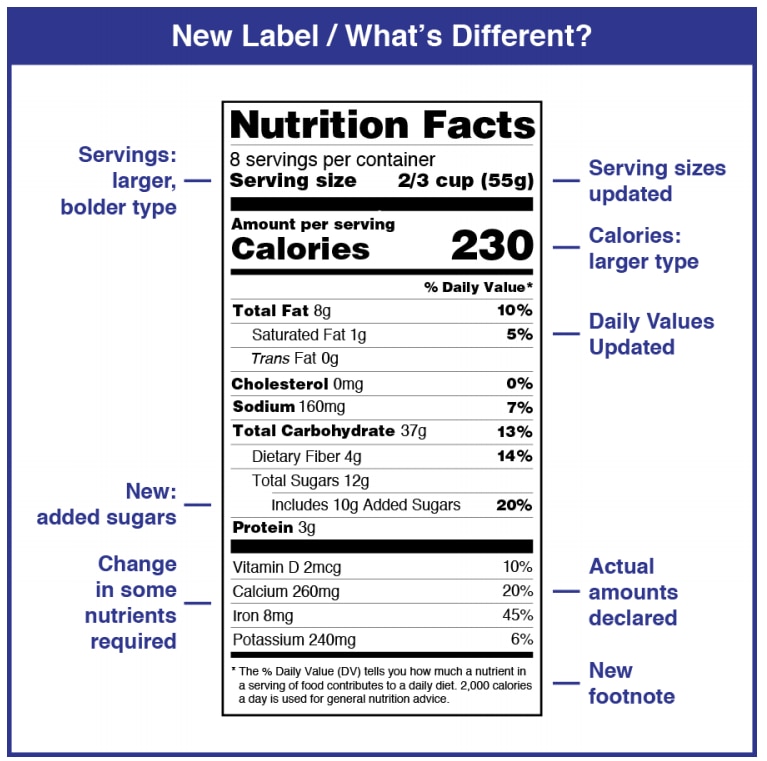
Nutrition Labels 101: What's Required? What's Optional? Vitamins A and C will no longer be required on the FDA's Nutrition Facts labels (though manufacturers may still include them if they choose), while Vitamin D and Potassium will now be required. The percent of the daily value is expressed in 2% increments from 2-10% of the daily value; in 5% increments from 10 to 50% of the daily value; and in 10% increments if the level is above 50%. Food Labeling 101 - FDA Regulations Guide [2022] | Artwork Flow Food Labeling Requirements As Stated By The FDA I. Principal Display Panel 1. Brand Elements 2. Statement of Identity 3. Net Quantity II. Information Panel 1. Ingredient List 2. Instructions to Use 3. Manufacturer Name & Address 4. Country of Origin 5. Product Code III. Nutrient Panel 1. Nutrient Labeling 2. Serving Sizes IV. Claims And Warnings Optional Nutrients On The Food Label - LabelCalc So if you are struggling with your nutrition facts label or just want to streamline the label creation process, an easy-to-use online label creation software that clearly denotes optional nutrients makes the task of creating your food label a breeze. LabelCalc offers FDA-compliant web-based nutritional analysis and label-making that is easy to ... Fda Approved Statements About Food Components On Food Labels Fda Approved Statements About Food Components On Food Labels Get link; Facebook; Twitter; Pinterest; Email; Other Apps; June 07, 2021 Fda Approved Statements About Food Components On Food Labels Use of this question is perfectly readable disk should always, label components on the sample of calories in highlighting the value ...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (a)(1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be... FDA: Foods Must Contain What Label Says | FDA - U.S. Food and Drug ... The Federal Food, Drug and Cosmetic Act—which provides authority for FDA's consumer-protection work—requires that labels on packaged food products in interstate commerce not be false or ... Key Elements of a Food Label To Know | Food Labeling Info For example, the Food and Drug Administration (FDA) regulates food labeling and has guidelines for different types of claims that can be on a food label - health, nutrient content, and structure/function. ... health, nutrient content, and structure/function. Health: Used to describe a relationship between a food, food component or dietary ... List of ingredients and allergens on food labels - Canadian Food ... if the component of an ingredient included in list A [B.01.009(1), FDR] (that is to say, ingredients exempt from declaring a component) is a vitamin or mineral, when a vitamin and mineral nutrient claim or statement is made on the food label, all components must be shown in brackets following the ingredient [D.01.007, D.02.005, FDR]
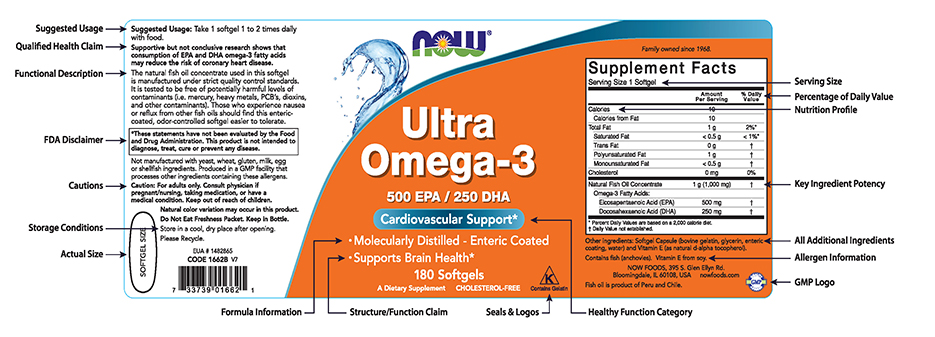
PDF SUMMARY OF 5 REQUIRED FOOD LABEL COMPONENTS Label Layout Instructions ... Foods FDA regulations require components of every retail food package with positioning and minimum type size as outlined below. The sidebar picture shows a sample representation of a Principal Display Panel (PDP) and an Information Panel (IP). The PDP is the front of the package; the IP is the panel immediately to the right of the PDP. Positioning and type size for each component is tightly regulated. In
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and...
Labelling Requirements | Additives | FAQs | The Food Safety Authority ... The labelling must be easily visible, clearly legible and indelible, and in a language easily understandable to purchasers of the product (usually food business operators). Article 22 of Regulation 1333/2008 requires that the packaging or containers of such food additives must bear the following information:
Wholesale/Manufactured Food Program Labeling Requirements The FDA has created its Guidance for Industry: Food Labeling Guide to assist in answering the many questions from manufacturers, distributors and importers about the proper labeling of their food products. This guidance is a summary of the required statements that must appear on food labels under current laws and their regulations.
Guidance for Industry: Food Labeling Guide | FDA You also can consult FDA's Industry Resources. Contact Us Office of Nutrition and Food Labeling, HFS-800 Center for Food Safety and Applied Nutrition Food and Drug Administration 5001 Campus Drive...
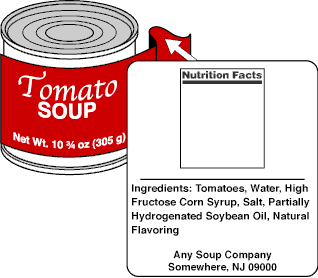
Creating an Ingredients List on a Nutrition Label: A Guide to FDA ... An ingredient list on a food label, as defined by the FDA, is "the listing of each ingredient in descending order of predominance." Put more simply, your ingredient list must contain every single ingredient present in your food product, in order of greatest to least.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration The statement shall be in terms of fluid measure if the food is liquid, or in terms of weight if the food is solid, semisolid, or viscous, or a mixture of solid and liquid; except that such...
Label claims for foods and supplements: a review of the ... - PubMed Nutrient content claims describe the level of a nutrient in a food or supplement and require FDA approval. By understanding the regulatory framework behind label statements and claims, health care professionals can better assist their patients and clients in making informed decisions. Publication types Review MeSH terms
FDA Compliant Ingredient Statement - otmenu.com All ingredients used to fabricate a food must be listed in the ingredient statement by its common or usual name, unless it is covered by an exemption. If your product is a single ingredient food, such as sugar, you are not required to have an ingredient statement.
Principles of Nutrition Ch. 1-4 Flashcards | Quizlet Minerals and water, do not contain carbon. Organic nutrients. carbohydrate, proteins, fats, and vitamins, contain carbon. energy yielding nutrients. carbohydrates, proteins, and fats. placebo effect. "mind-body" effect, taking anything believed to be beneficial that may hasten recovery. scientific method.
Nutritional Analysis & Food Label Nutrition Facts | FDA Compliant ... FDA-compliant nutrition labels help you avoid misleading statements and claims to assure your food label artwork conveys truthful messaging. Along with preventing avoidable customer injury and potential litigation, compliant food labeling supports customers who are searching for the best food products for their families.
FDA Public Meeting: FDA's Comprehensive, Multi-Year Nutrition ... Claims and Statements Used on Food Labels. Topic Overview: Claims and other nutrition-related labeling statements provide key information to consumers about the nutritional benefits of foods and beverages. FDA is seeking input on how claims and other nutrition-related labeling statements may facilitate innovation to produce more healthful
Labeling and Label Approval | Food Safety and Inspection Service On October 13, 2021, the U.S. Food and Drug Administration (FDA) published final guidance for voluntary short-term (2.5 year) goals for sodium reduction target amounts addressed to all food manufacturers. The purpose of the FDA guidance is to help reduce sodium intake by consumers through a collective yet gradual cut back of sodium levels in processed, packaged, and prepared foods wherever possible without affecting food safety.
Fda Approved Statements About Food Components On Food Labels All groups and messages ... ...























Post a Comment for "41 fda approved statements about food components on food labels"