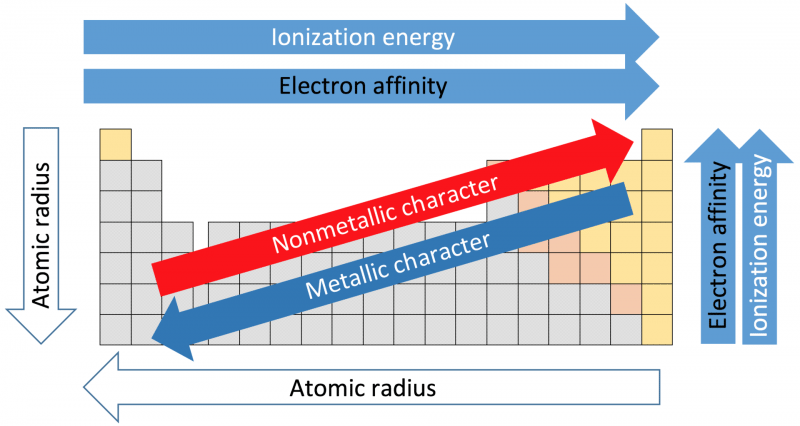
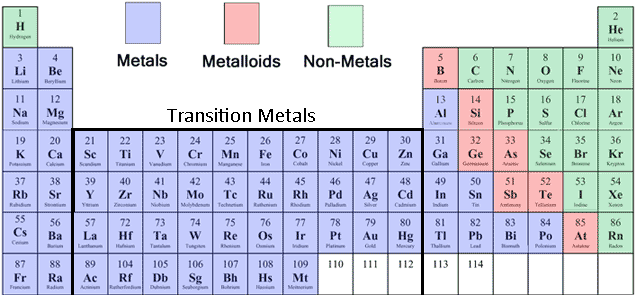
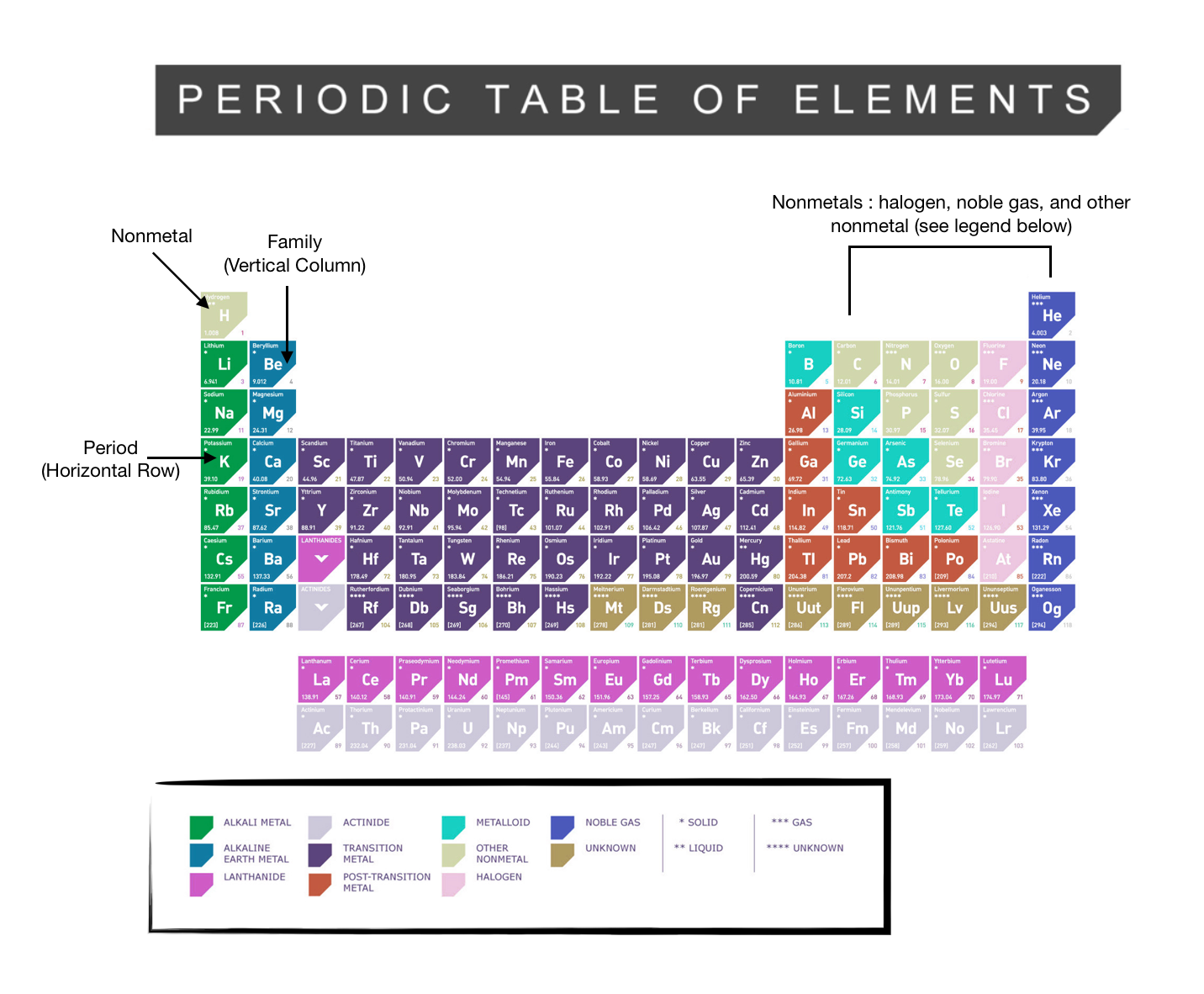
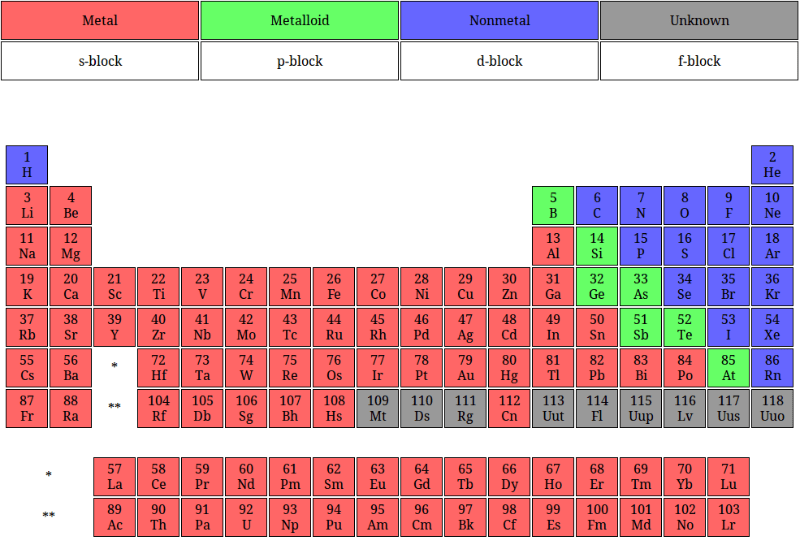
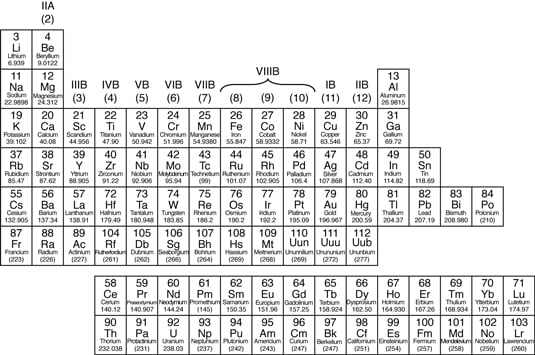
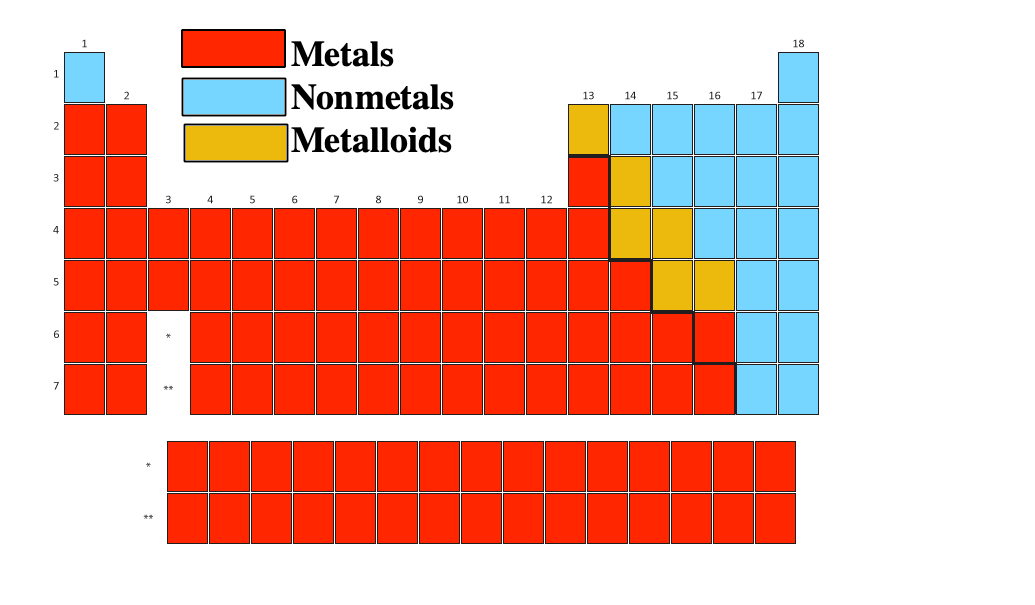
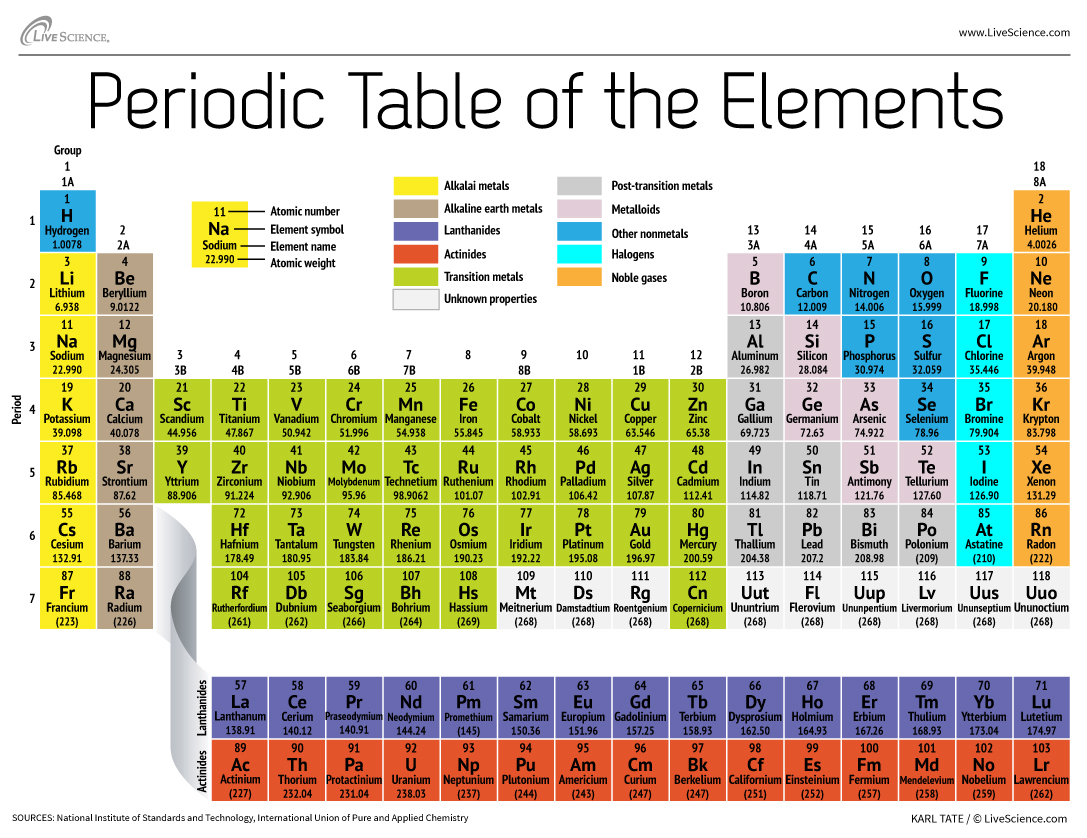
38 periodic table with metal and nonmetal labels
quizlet.com › 334188644 › chem-ch-2-flash-cardsChem Ch 2 Flashcards | Quizlet A main group metal tends to lose electrons, forming a cation with the same number of electrons as the nearest noble gas in the periodic table. A main group nonmetal tends to gain electrons, forming an anion with the same number of electrons as the nearest noble gas. The various groups gain or lose electrons as summarized in the following table: › 39339278 › General_Chemistry_9thGeneral Chemistry 9th-Ebbing.Gammon - Academia.edu Enter the email address you signed up with and we'll email you a reset link.
chemapps.stolaf.edu › jmol › docsJmol/JSmol Interactive Script Documentation - St. Olaf College Atoms for these file types can be selected using these names, and the names can be displayed in labels using the format code %a. For file types such as XYZ that do not indicate a number with the atom symbol, Jmol constructs an atom name from the element symbol and the sequential number of the atom in the file.

Periodic table with metal and nonmetal labels
› Full_MembersFull Members | Institute Of Infectious Disease and Molecular ... Full member Area of expertise Affiliation; Stefan Barth: Medical Biotechnology & Immunotherapy Research Unit: Chemical & Systems Biology, Department of Integrative Biomedical Sciences quizlet.com › 160124557 › chem-ch-3-chem-ch-2-flashChem Ch 3, Chem Ch 2 Flashcards | Quizlet A main group metal tends to lose electrons, forming a cation with the same number of electrons as the nearest noble gas in the periodic table. A main group nonmetal tends to gain electrons, forming an anion with the same number of electrons as the nearest noble gas. The various groups gain or lose electrons as summarized in the following table: en.wikipedia.org › wiki › Periodic_tablePeriodic table - Wikipedia The periodic table is a graphic description of the periodic law, which states that the properties and atomic structures of the chemical elements are a periodic function of their atomic number. Elements are placed in the periodic table by their electron configurations , [17] which exhibit periodic recurrences that explain the trends of ...
Periodic table with metal and nonmetal labels. en.wikipedia.org › wiki › NonmetalNonmetal - Wikipedia In periodic table terms, an analogy can be drawn between the noble gases and noble metals such as platinum and gold, with the latter being similarly reluctant to enter into chemical combination. As a further example, xenon, in the +8 oxidation state, forms a pale yellow explosive oxide, XeO 4 , while osmium , another noble metal, forms a yellow ... en.wikipedia.org › wiki › Periodic_tablePeriodic table - Wikipedia The periodic table is a graphic description of the periodic law, which states that the properties and atomic structures of the chemical elements are a periodic function of their atomic number. Elements are placed in the periodic table by their electron configurations , [17] which exhibit periodic recurrences that explain the trends of ... quizlet.com › 160124557 › chem-ch-3-chem-ch-2-flashChem Ch 3, Chem Ch 2 Flashcards | Quizlet A main group metal tends to lose electrons, forming a cation with the same number of electrons as the nearest noble gas in the periodic table. A main group nonmetal tends to gain electrons, forming an anion with the same number of electrons as the nearest noble gas. The various groups gain or lose electrons as summarized in the following table: › Full_MembersFull Members | Institute Of Infectious Disease and Molecular ... Full member Area of expertise Affiliation; Stefan Barth: Medical Biotechnology & Immunotherapy Research Unit: Chemical & Systems Biology, Department of Integrative Biomedical Sciences









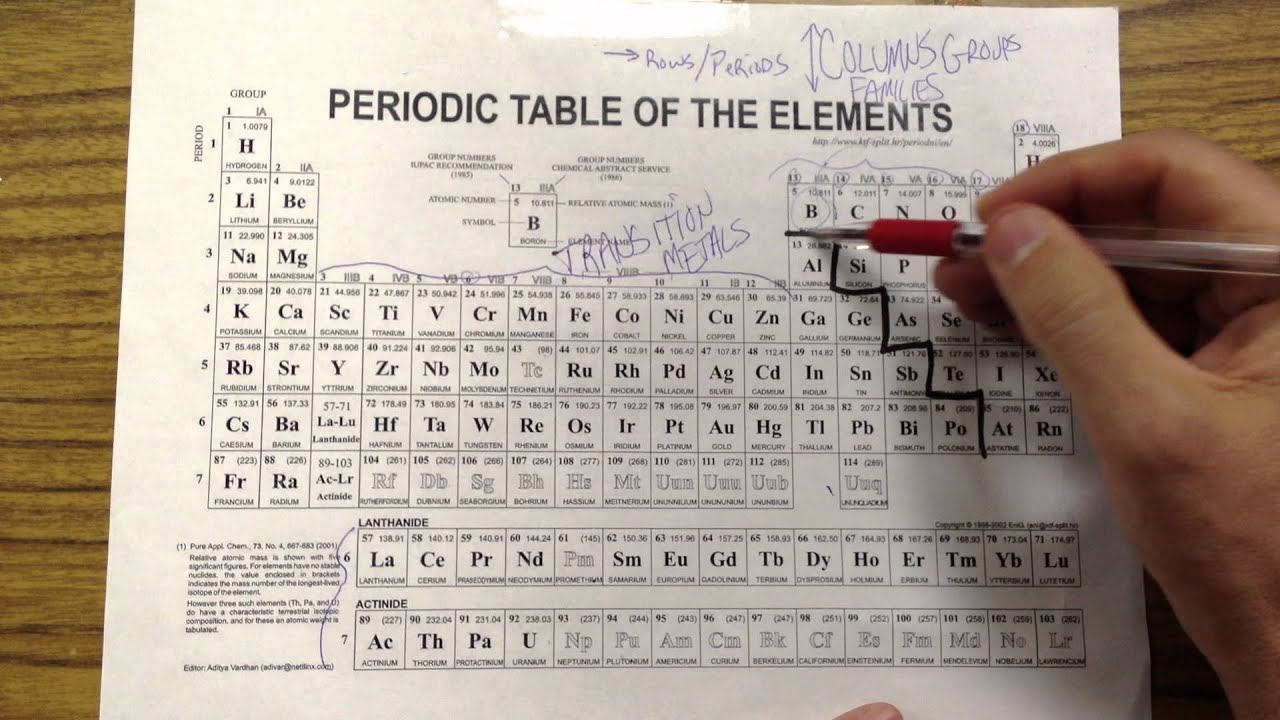
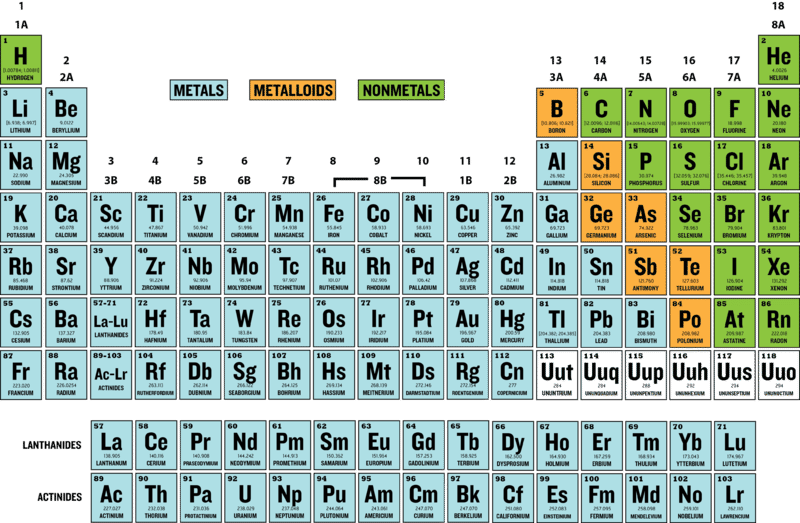


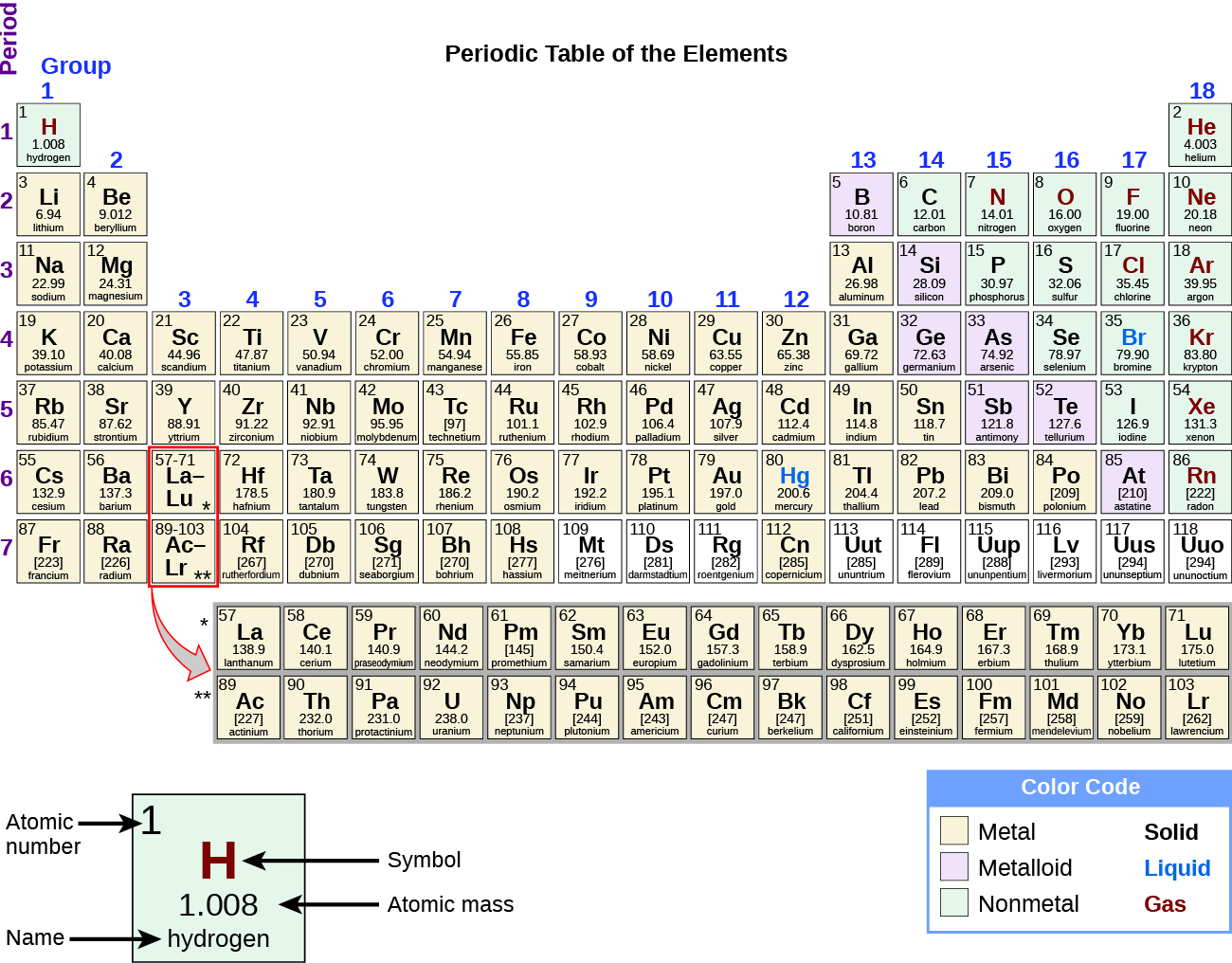
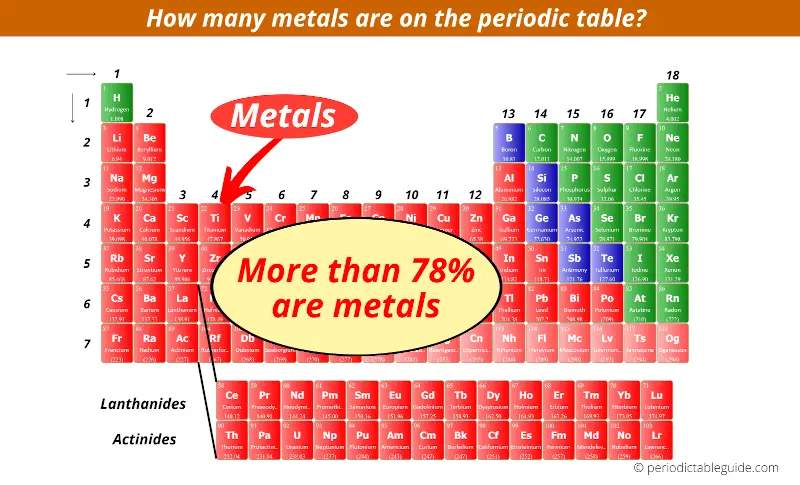

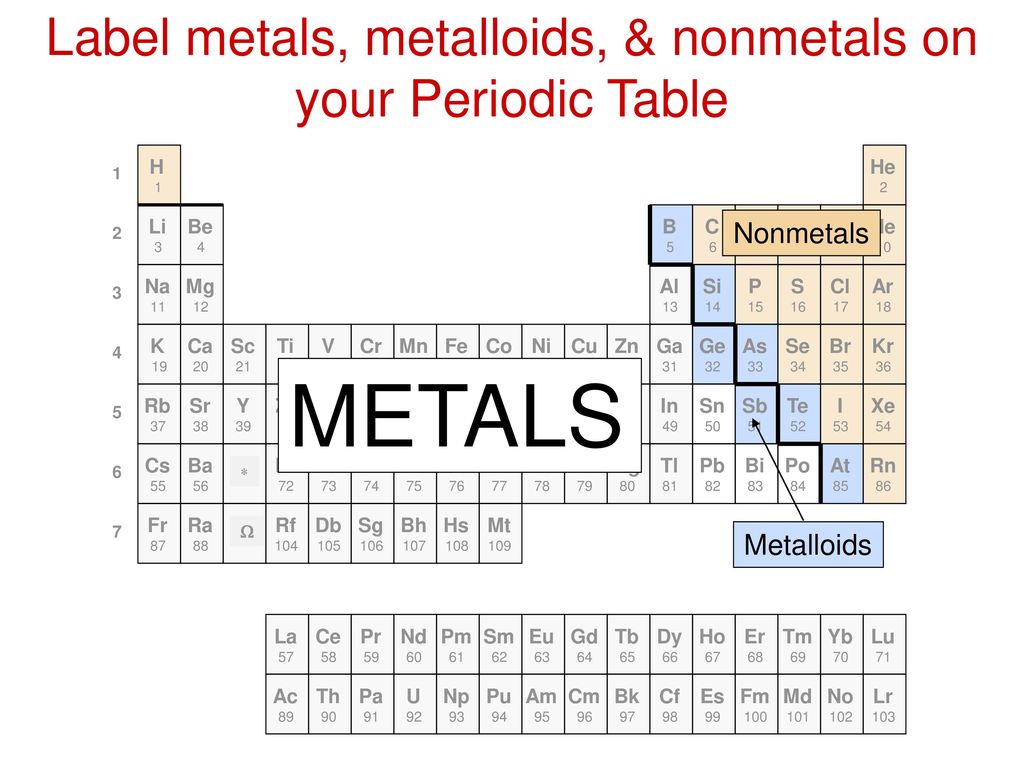
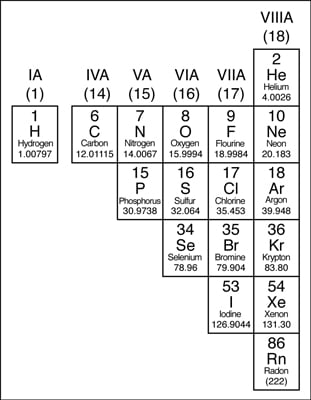



Post a Comment for "38 periodic table with metal and nonmetal labels"